Why Clinical Trial Costs Are Skyrocketing
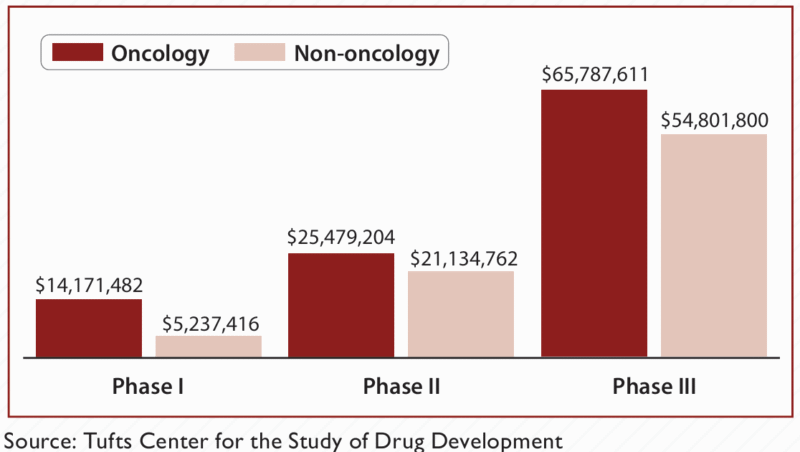
Clinical trials sit at the heart of drug development, but their budgets are climbing faster than ever—with Phase III trials now frequently costing between $20 and $100 million. A big chunk of these costs stems from the sheer size and scope of patient recruitment and monitoring. When the wrong patients are enrolled or efficacy signals are weak, trials get longer and more expensive, and many ultimately fail to produce convincing results. In the modern world of biologics and CAR-T therapies, which are designed for very specific patient populations, relying on broad-based enrolment just doesn’t work anymore.
Enter biomarker-driven patient selection: by focusing on enrolled patients most likely to respond, sponsors can trim trial size, boost efficacy, get answers faster, and save millions in the process. 1
The Hidden Risks of Inefficient Patient Selection in Trials
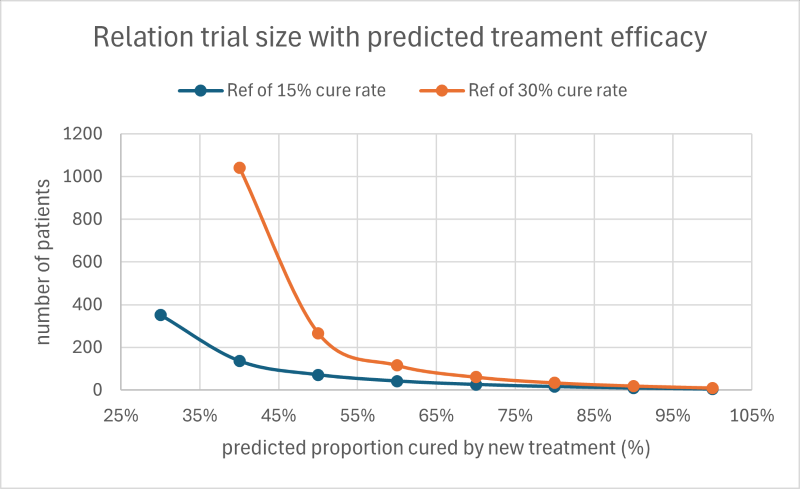
Accurate trial sizing is critical—statisticians carefully calculate the minimum number of patients needed for “statistical power,” but enrolling too many or the wrong kinds of patients drives up cost and complexity. For example, increasing efficacy by just 10% via better patient preselection can slash required enrolment by up to half.
Large, unfocused trials can be financial sinkholes: average Phase III costs reach $20-100 million, with patient recruitment accounting for up to 40% of that, and every additional patient means longer studies and less time before patent expiration. 2
Industry studies show only 10–15% of traditional Phase I–III trials succeed, and poor patient stratification is often to blame. This failure rate is even higher for personalized therapies like CAR-T, where costs can reach $1–2 million per patient. 3
Delays are another silent threat: each day a Phase III trial drags out can cost hundreds of thousands of dollars, and patient dropout rates (15–30%) mean sponsors must keep recruiting just to hit statistical goals.
How Biomarkers Change the Math for Patient Selection
Biomarker-based patient selection—also known as patient stratification—allows sponsors to:
- Recruit only likely responders, shrinking trial sizes by 30–50%.
- Ramp up efficacy signals, increasing odds of regulatory approval.
- Speed up study timelines, cutting operational costs and getting therapies to market up to a year sooner. 4
This “precision medicine” advantage means more confident results, smaller budgets, and increased chance of clinical and commercial success.
Real-World Example #1: Type I Interferon (IFN) Signature in Lupus Biologic Trials
Systemic lupus erythematosus (SLE) is notoriously tough to tackle—patients’ symptoms and underlying biology vary widely, making traditional, broad clinical trials prone to disappointing results. Often, many enrolled participants don’t even have the disease mechanism a new drug targets, diluting drug efficacy and raising placebo response rates. 5
The turning point came when researchers identified the IFNα gene signature as a predictive biomarker for anti-IFN biologics like anifrolumab. In landmark TULIP-1 and TULIP-2 studies, lupus patients with strong IFNα signatures had significantly higher response rates (~47.6%) to anifrolumab compared to those without (~28.2%). 6
Using this biomarker allowed research teams to build trials with smaller, more focused cohorts, accelerating study timelines and increasing the clinical impact. By matching patients to therapies targeting their unique disease biology, IFNα biomarker selection strengthens efficacy signals, lets trials be smaller and cheaper, and advances care by making SLE trials more scientifically rigorous and personalized. Peer-reviewed clinical data now supports this approach as the gold standard for future lupus drug development. 7
Real-World Example #2: PD-L1 as a Biomarker in Lung Cancer Trials
Non-small cell lung cancer (NSCLC) patients used to get a “trial and error” approach—standard drugs first, with targeted therapies reserved for later. The problem: without biomarker testing (EGFR, ALK, ROS1, PD-L1, etc.), many patients received therapies that didn’t fit their tumour’s molecular makeup, leading to shorter survival, more side effects, and wasted resources.
In pivotal pembrolizumab (Keytruda) trials like KEYNOTE-024, only patients with PD-L1 “tumour proportion score” ≥50% could enrol. The result? Overall survival doubled for PD-L1-high patients, and trial sample sizes were cut by up to half. 89
Cost-effectiveness modelling shows biomarker-driven trials can lower clinical budgets by 30–50%, with savings of $15–50 million per Phase III study. 10
This approach means fewer unnecessary treatments, faster answers, and a stronger case for regulatory approval.
Key Takeaways
- Precision patient selection dramatically improves trial efficiency and success, maximizing every dollar spent.
- By focusing on the right patients, sponsors get better results, save on recruitment and monitoring, and speed up time to market.
- Regulators increasingly expect trials and launches to use robust biomarker strategies, so building companion diagnostics into the plan is crucial.
- Smaller trials that deliver bigger results are attractive to payers, investors, and partners, supporting better commercial outcomes and competitive differentiation.
Bottom line: investing early in biomarker-driven strategies lets biotech companies “fail fast, fail cheap”—or succeed faster, with stronger data, less risk, and more healthcare impact.
Footnotes
- Tumor biomarkers for diagnosis, prognosis and targeted therapy | Signal Transduction and Targeted Therapy ↩︎
- Mar-Apr-2022 Clinical Trial Budgets | Clinical Trial Vanguard FD5: Phase II Trials in Drug Development and Adaptive Trial Design – PMC ↩︎
- ASCO Educational Book (CAR-T) ↩︎
- Comparison of efficacy discrepancy between early-phase clinical trials and phase III trials of PD-1/PD-L1 inhibitors – PMC ↩︎
- Worldwide Clinical Trials SLE White Paper FD11: NEJM Anifrolumab SLE Trial ↩︎
- NEJM Anifrolumab SLE Trial FD12: White B et al. Arthritis Rheumatol SLE Biomarker ↩︎
- White B et al. Arthritis Rheumatol SLE Biomarker ↩︎
- Scientific Reports, Background and clinical significance of biomarker-based patient enrichment in NSCLC drug development ↩︎
- JITC, Determinants of 5-year survival in NSCLC with PD-L1 ≥50% ↩︎
- Frontiers, Integrating treatment cost reduction strategies and biomarker research pmc.ncbi.nlm.nih+2: NSCLC biomarker use ↩︎
