Accelerated Trastuzumab clone selection by early all-in-one insights into yield, Fab & functional Fc affinity, confirmed by structural data.
The continuous analytical waltz toward the optimal antibody candidate
Lead optimization is an iterative and complex process involving cycles of design, production, purification, and characterization. As development progresses assays become more time-consuming, costly, and technically demanding – making early, high-quality decisions critical to avoid late-stage failures.
Traditionally, Fab target binding, Fc-receptor interactions, and structural integrity are assessed in separate phases. This fragmented approach can obscure true high-performing candidates or reveal liabilities only later, optimizing wrong candidates and thus increasing the number of candidates carried forward unnecessarily.
Imagine you get more critical information from all your candidates earlier on
In this study on Herceptin variants, RIC and Vixen evaluated four clones alongside the originator and a degraded reference using a data-rich, integrated approach. This method enables early assessment of yield, FcRn binding potency, and Fab target affinity, combined with built-in isolation for direct structural MS analysis.
This revealed hidden post-translational modifications impacting functional potency – issues typically detected only in later optimization stages. By linking yield and affinity data with glycoform analysis, structural risk assessment, and peptide mapping, development decisions can be guided with greater precision and confidence.
Ultimately, this approach enables confident early candidate selection, reducing unnecessary downstream engineering and production cycles and focusing resources on true high-performing candidates.
Streamlined workflow for Herceptin clone selection
Herceptin (commercial name Trastuzumab) is the originator monoclonal antibody therapeutic targeting Human Epidermal Growth Factor Receptor 2, otherwise known as “HER2”. HER2 stimulates cell proliferation and is over expressed on certain cancer cells and serves therefor as a powerful target for immunotherapy in certain breast, ovarian, and gastric cancers.
Vixen Bio’s lead optimization assay enables an integrated all-in-one solution directly from supernatant:
- At-line titration with direct Fc-receptor feedback
- Fab target binding affinity measurement, with feedback on stability
- Direct isolation of mAb candidates for downstream analysis
- Mass spectrometry confirms the affinity results and structural integrity
Study insights
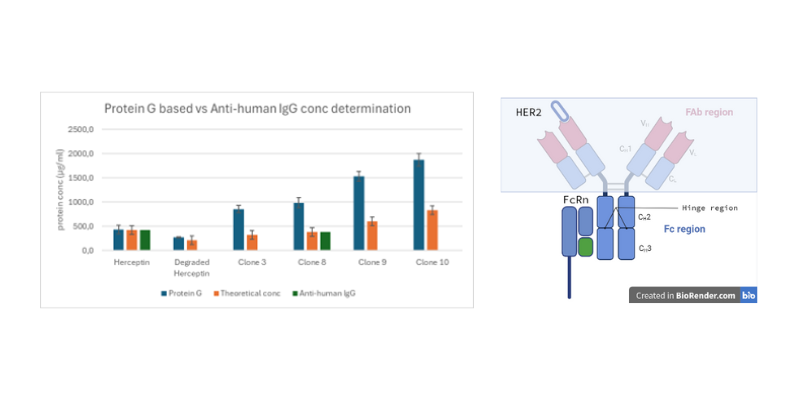
Yield & Early Fc-functionality
Quantitation from the medium yielded label-free, accurate concentrations using a calibration curve. Protein G Fc-capture, which binds the CH2–CH3 hinge region, provided direct feedback on Fc structural changes affecting Fc receptor binding (such as FcRIIa, FcRIIIa, FcRn and complement activation) when compared with anti-hIgG capture. Antibody capture measured not only titer but also the effects of PTMs at the CH2–CH3 hinge region, unlike the anti-hIgG method, which is insensitive to structural changes in this region.
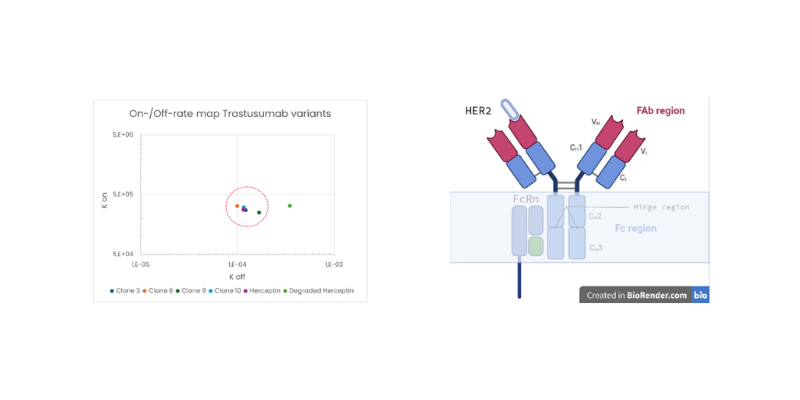
Kinetic ranking
The following kinetic profiling of mAb’s Fab binding to HER2 showed that all clones have a comparable kon, koff and KD values compared to Herceptin, as seen in the On-/Off-rate map. The degraded version exhibited reduced affinity caused by a significant higher koff, indicating that affinity can be applied as measure for stability validation.
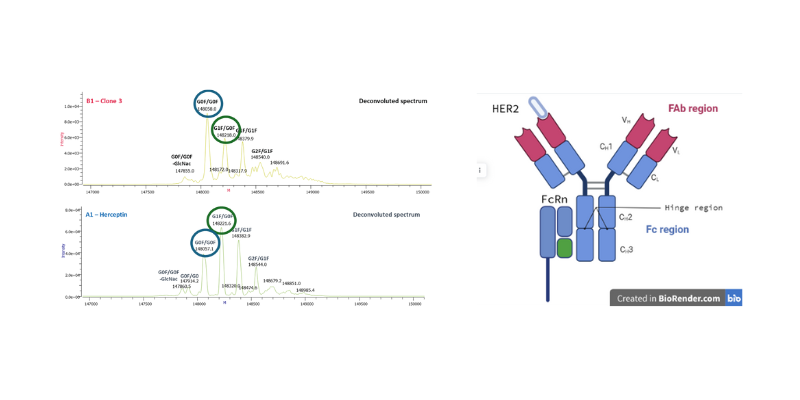
Total structure analysis by MS
Mass spectrometry on isolated fraction by White FOx confirms structural integrity in molecular weights and glycoform distribution. The PTM’s that translate in affinity shifts on the FcRn-region were subsequently identified by peptide mapping as a decrease in galactose distribution. This leads to a confirmed functional impact by decreased affinity for Fc receptors such as FcRIIa, FcRIIIa, FcRn and complement activation.
Integrated early-stage analytics across all functional domains: reducing risk, time, and cost
This methodology enables comprehensive early-stage characterization across functional domains, providing robust, data-driven insights into antibody performance. By delivering complete functional profile insights at an early stage, it significantly increases the understanding of your designs and processes and thus reduces the number of candidates advancing and the need for multiple optimization rounds, efficiently de-risking the lead optimization process and maximizing return on R&D investment.
- At-line titration with direct feedback on the crucial FcRn binding region → Supports selection for desired mAb half-life and avoids PTMs with functional impact
- Full kinetic profiling of target binding efficacy → Identifies stability issues and enables selection of the desired kinetic profile
- High-purity isolation with flexible yield control → Provides material ready for downstream analyses
- Structural integrity confirmation of top-performing candidates only → Reduces unnecessary optimization rounds and a secured filing procedure.
Interested in learning how our personalized assays can help streamline your optimization rounds? Let’s connect and discuss how we can support your work.
Interested in more details? Download the white paper below.
