“From buffer to Patients: Affinity assay predicting therapeutic behaviour in patients”
Why Measuring Affinity in Native Plasma Matters for Therapeutic Development
Affinity defines how strongly a therapeutic antibody binds to its target — and it is one of the most important parameters influencing efficacy, dosing, and clinical performance.
Traditionally, affinity is measured in purified systems. These controlled conditions allow us to understand binding at a basic level, but they often fail to capture the complexity of the therapeutic’s true environment: human plasma.
Our study case hypotheses and underscores the importance of screening in context during drug development. All the right physicochemical data can be present, but the therapeutics’ behaviour could still be very different in the patient. In case of a biosimilar, which typically rely on comparability filing without clinical trials, the clinical outcome might unexpectedly not match expectations on efficacy or worse, safety.
The Case for Native Environments
Plasma is where biologics function. It’s also a complex matrix of proteins, receptors, and other components that can influence how a therapeutic behaves. Measuring affinity here provides insights that purified systems don’t show.
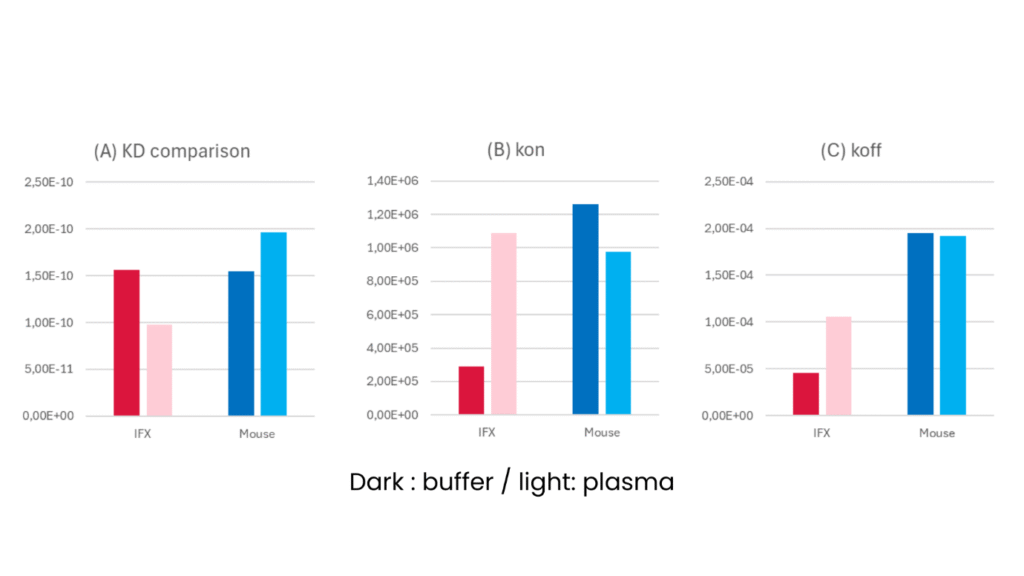
For example, our case study on antibodies binding to TNFα: infliximab (IFX) and a mouse anti-TNFα antibody, comparing binding kinetics in purified versus plasma conditions. Both IFX and a mouse anti-TNFα antibody showed similar equilibrium constants (KD) in purified assays (dark columns in figure 2A , the kinetics in plasma told a different story ( lighter columns in figure 2):
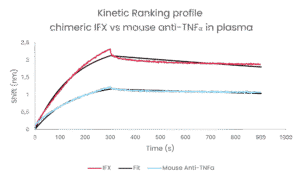
- An ELISA like method would perfectly match the performance of these 2 antibodies. Actually measuring the kinetics already shows a different binding and releasing speed, but the mouse antibody faster binding is compensated by its faster release. This implies the mouse antibody would have a rapid effect, but needs more frequent or higher dosing.
- In plasma however, Infliximab showed a significant improved association rate compared to purified conditions. While the mouse antibody performed similar or slightly worse.
- Infliximab showed a slight decrease in dissociation rate while the mouse anti-TNFα antibody remained exactly the same.
- This results in Infliximab showing better KD and thus higher efficacy in the patient. While the mouse alternative becomes less performant.
This difference highlights why measuring affinity in plasma matters: it reveals how candidate therapeutics may truly interact with their targets in vivo – where their function matters most. The buffer conditions used in classic SPR may not accurately reflect the physiological-binding environment in terms of pH, temperature, or composition. Weak interactions between therapeutic antibodies and serum components could impact the biophysical and pharmacological properties of therapeutic antibodies.
Additionally, by typing the therapeutic antibody with plasma, it can serve as an early indication towards expected efficacy as affinity can be quite different between buffer and plasma.
If a therapeutic is developed without plasma affinity risks are the efficacy could easily differ a factor 2 or more in the actual clinical trial requiring major not understood adaptations to the PK/PD modelling.
If a biosimilar is developed with just the required data in buffer showing good comparability, an ineffective or unsafe therapeutic might make it to market.
Impact Across the Development Pipeline
Native affinity measurements bring value throughout the therapeutic lifecycle:
- Engineering: Mapping Fab and Fc affinity in conditions that reflect reality -supports better candidate selection.
- Preclinical: Building the right data for regulatory filing by gaining insight into mechanism of action, clearance pathways, and dosing in donor plasma.
- Clinical: Strengthen therapeutic drug monitoring (TDM) and immunogenicity assessments by linking personalized kinetics to functional outcomes.
- Commercialisation: Reduce risk by ensuring therapeutic performance holds up beyond simplified assay conditions.
Vixen Bio’s Expertise
At Vixen Bio, we specialise in complex affinity studies (epitope and Fc) — not just in purified systems, but in the environments where your therapeutics must ultimately perform.
By applying advanced analytical approaches to your samples under physiologically relevant conditions, we help you:
- De-risk development early, by providing the relevant data in the right context
- Accelerate your path from discovery to commercialisation, by supporting a substantiated NDA or comparability filing.
Key takeaway: Affinity in purified systems gives you part of the picture. Affinity in plasma gives you the real one.
👉 If your program depends on getting binding right, let’s explore how native and patient-relevant studies can support your success.
