Expanding Capabilities in Antibody Detection: Launch of Our New Protein G Biosensor Probe
We are excited to announce the release of our new Protein G biosensor probe, an important expansion of our biosensing portfolio. Complementing our existing Protein A probe, this new tool broadens antibody subclass and species detection with high sensitivity, reproducibility and specificity.
Key advantages:
- Fuller coverage of antibody species and subclasses for all development needs and cross- species research.
- Highly regeneratable with excellent surface recovery and reproducibility
- Wide dynamic range (subclass dependent)
- Allows for at-line titration and screening on affinity
Extending coverage, extending possibilities
Protein A and Protein G are both bacterial proteins widely used as anchoring tools in purification and quantification assays. They share key characteristics, including:
- Recognition of antibodies through the Fc region of the heavy chain
- High resilience to acidic regeneration conditions
- Stable binding capacity over multiple cycles
The key differences can be found in their inherent affinity they showcase towards different antibody species and subclasses. With broad and complementary binding profiles, Protein G binds strongly to IgG antibodies from a wider range of species -including human, mouse, rat, rabbit, cow, horse, and goat. This makes Protein G particularly valuable for cross-species research and therapeutic antibody development.
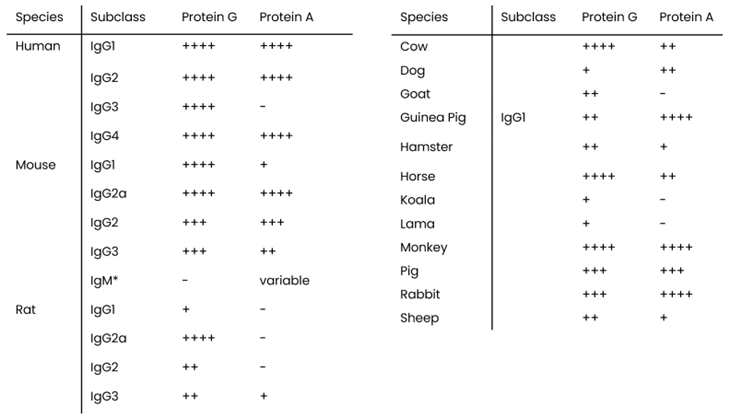
Same species & subclass, different affinity – the effect of glycosylation
Even when working with the right antibody species or subclass, glycosylation patterns can significantly impact recognition sites for Protein A and Protein G. These subtle differences affect affinity and binding behaviour!This dynamic duo allows for direct affinity screening and at-line titration before purification and allows you to anticipate on these subtle differences. This not only supports optimal (column) purification strategy selection, but also directly allows to improve yield while providing early insights in structural integrity.
Key Features and Capabilities
Now, let’s explore the impressive specifications that make this innovative solution truly stand out. While designed to provide you the cross-species compatibility and extending detection possibilities, we didn’t lose sight of top-quality requirements! High reproducibility & sensitivity are key properties, allowing for robust, accurate and precise determinations. Since the sensors work directly in crude sample types, it allows for rapid titre screening without any required prior purification.
In one evaluation, the binding capacity of a rat IgG2a demonstrated:
- A dynamic range spanning ~4 logs of concentration*
- A precise linear range from 0.1 µg/ml to 6.25 µg/ml
- Exceptional reproducibility (CV% = 4.4% across 20 regeneration cycles)
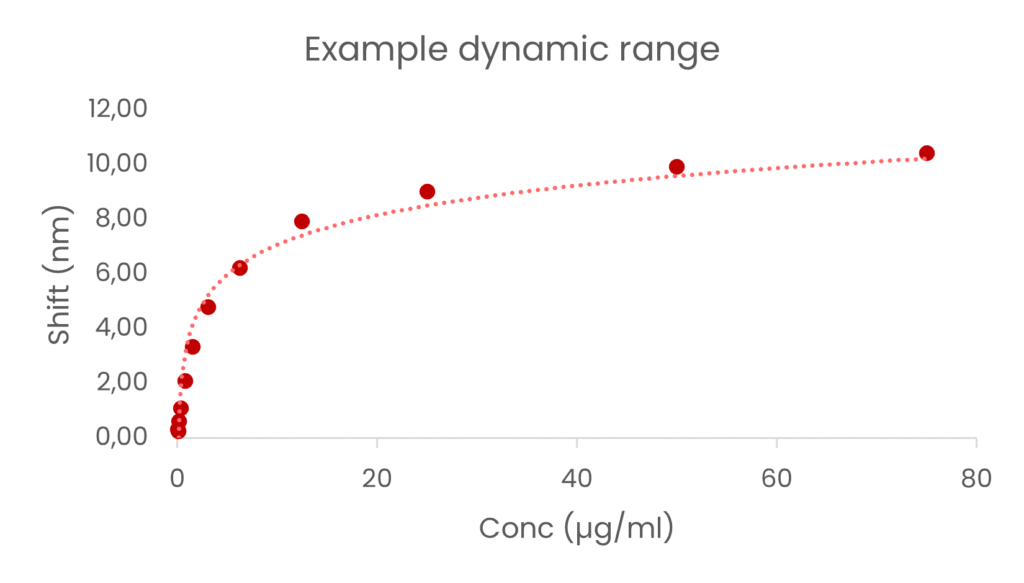
*Differences in range can be expected with different species & subclass
Enabling Better Research and Therapeutic Development
The synergy in affinity between Protein A and Protein G means researchers can now select the probe -or combination of probes- that best fits their experimental needs, improving confidence in antibody characterization, quantification, purification and interaction studies.
In short, the release of our FO-SPR Protein G biosensor is poised to accelerate workflows in areas such as:
- Discovery & Development: Antibody purification optimization and characterization
- Preclinical Studies: Cross-species antibody screening
- Quality Control: Robust quantification during therapeutic antibody production
Together, our Protein A and Protein G probes deliver unmatched flexibility and reliability in antibody detection -helping you move from discovery to manufacturing with greater accuracy and efficiency.
Ready to learn more?
Stay tuned for our upcoming application note, where we’ll share real-world examples of how the Protein G biosensor probe enhances antibody workflows.
